1. Keep the battery out of reach of children in a well lit, well-ventilated place.
2. Easy accessibility to the battery location is important in order to ensure routine servicing like water topping, terminal cleaning etc.
3. Never short circuit the battery terminals as it can cause major fire. Always keep the terminal covers in place to avoid short circuit.
4. Do not take out electrolyte from the battery by user, as the sulfuric acid contained in the same can cause body burns. Spilling electrolyte on the floor will damage your floor.
5. Do not dispose the old battery to a non-authorized person. As per Govt. of India Notification, “It shall be the responsibility of the consumer to ensure that used batteries are not disposed of in any manner other than depositing with the dealer, manufacturer, re-conditioner or at the designated collection centres”
1.Battery Charging
POWERCEL C10 rated tubular batteries are recommended to be charged at amperes of 6% to 8 % of the battery AH capacity. Maximum charging current must never exceed 10% of the battery capacity. For example, the maximum charging current for a 100 AH battery must be less than 10 amps. Preferred charging current 6 to 8 amps.
Charging upper cut off voltage is to be 14.5 Volts and discharging lower cut off voltage should not be less than 10.6 Volts. Setting a discharge lower cut off voltage of 10.8 Volts for a 12 Volts battery will give much longer life for POWERCEL batteries.
[CAUTION] : Never keep the battery in discharged condition for long periods ( one week and above) as it can cause formation of sulfation and hardening of plates. This will cause battery capacity loss (back up loss) and can even lead to premature battery failure.
2. Top up the battery with battery grade distilled water once in 5 to 6 months or whenever the electrolyte level indicator float nears the lower red marking of the vent plug tube.
[CAUTION] Never add Acid as it can accelerate grid corrosion
[CAUTION] Never allow the battery electrolyte level to fall below the top of the battery plates as it can cause irreversible plate damage due to battery heating up. This will result in capacity loss and premature failure of your battery.
3. Ensure that the battery terminals and the connection tags are clean and without corrosion. Clean the battery cover top and terminals with a wet cloth periodically. Vaseline or Petroleum jelly may be applied to terminals which will help avoid corrosion. A clean battery will prevent the chances of surface leakage self-discharge.
[Caution] Before cleaning the batteries, switch off the Inverter, UPS and the connected equipments. Also take good care not to short circuit the terminals as it can cause sparking and major fire.
4. Clean the Vent Plugs in lukewarm water after unscrewing them from battery, once every year or whenever the same is seen to be covered with dust. Clean and unclogged vent plugs ensure that the hydrogen gas generated during the charging – discharging cycle goes out of the cell.
[Caution] If gases accumulate in the battery cells due to blocked vent plugs, it will lead to excessive pressure build up in the cells and can cause bursting of the container. Following a burst, electrolyte can spill over the storage area and nearby places and can damage your floor and nearby equipments.
The lead-acid battery, a reliable and quality technology, was invented by French physicist Gaston Planté in 1859. Remarkably, even after 150 years, Planté’s technology forms the foundation of modern-day lead-acid batteries.
The original design comprised two electrodes: a positive electrode and a negative electrode made of lead dioxide and metallic lead. These electrodes are separated by a rubber insulators and the assembly is immersed in dilute sulfuric acid electrolyte. The process of electricity generation begins with the emission of electrons by the positive anode during discharging. These electrons move to the negative cathode through the sulphuric acid electrolyte medium. From the cathode, the electrons and their accompanying charge moves to the external electrical load such as a light bulb, resulting in the flow of electricity.
A typical lead-acid battery cell develops 2 volts. By combining six such cells into a battery, an output of 12 volts is achieved, which is extensively used in today’s automobiles and various electrical and electronic devices. There are also 6 volt batteries with 3 cells which are mostly used by two wheeler industry. 2 Volt Single cell heavy duty batteries are extensively used in telecom industry, railways etc.
One of the most notable features of the rechargeable battery is the reversibility of the chemical reaction. By supplying DC electricity from an external source through the positive and negative terminals, the battery can be recharged to full capacity.
Deep cycle lead-acid batteries typically have a lifespan of about 1000 charging-discharging cycles at more than 50% depth of discharge. Automotive batteries, which are subject to shallow discharge, typically last 3 to 5 years, while industrial batteries can last from 5 years to as much as 7 to 8 years or more.
To achieve shallow but fast and high discharge capability, automotive batteries are manufactured with positive and negative electrodes consisting of a lead oxide pasted on lead alloy grids. In contrast, industrial batteries use tubular positive plates and pasted negative plates, with lead oxide for the positive filled in tubular bags. This design gives them the ability for deep discharge, durability and higher energy storage capacity.
Lead-acid batteries come with a host of advantages. They are cost-effective compared to other types of batteries, offering the lowest cost per KWH of energy storage. With over 150 years of development cycle, these batteries have proven to be rugged and reliable. Their robustness allows them to withstand high levels of abuse.
These batteries are also tolerant to overcharging and have a low internal resistance. They are capable of delivering very high currents and can be left on a trickle or float charge for extended periods.
Lead-acid batteries are available in a wide range of sizes and capacities. They can function in extreme environmental conditions and are easy to maintain, which contributes to their longer lifespan.
One of the significant benefits of lead-acid batteries is their recyclability. They are nearly 100% recyclable, which gives them a high resale value. This feature also makes them a green and environmentally friendly product.
Lead acid batteries are broadly grouped into two categories, based on the discharge current characteristics.
1. Automotive Batteries
They are capable of delivering high current for short durations ( ex: 2C to 5C current for 5 to 6 seconds), like the starter battery used in automobiles. They have more number of pasted thin positive and negative electrode plates that maximize reaction surface area and hence deliver higher rate of electrical current. However, they cannot withstand high discharge currents for long duration without the electrode plates getting damaged.
2. Deep Discharge Batteries
Deep discharge batteries are further sub classified in to 1. Pasted thick plate batteries and 2. Tubular Batteries.
Pasted Deep Cycle Batteries
Pasted Deep Cycle Batteries have much thicker plates than automotive batteries. They deliver less intense electric current ( 0.05C to 0.3C current), but for longer periods like many hours. These are the type of batteries many times found in UPS systems, Home Inverters, Fork lifts, Railway vehicle lighting, Solar electricity storage, Wind mill electricity storage, low-cost electric vehicles etc.
Tubular batteries
Tubular batteries use ribbed tubular bags for storage of positive electrode active material. Their applications are also same as what is mentioned for thick plate deep discharge batteries. The speciality of tubular batteries is that they are much more sturdy and durable than the thick plate deep discharge batteries and hence give much longer life.
VRLA (Valve-Regulated Lead-Acid) batteries
VRLA batteries belong to the deep discharge class batteries. They are further sub-classified as Gel batteries and AGM (Absorbed Glass Mat) batteries. They have different internal structures for their positive and negative plates.
VRLA batteries use pasted plates for both positive and negative electrodes. These plates consist of lead dioxide (PbO₂) for the positive plate and sponge lead (Pb) for the negative plate. The electrolyte is immobilized by a Gel or absorbed in a glass mat, preventing it from freely flowing within the battery.
Gel Batteries
Gel batteries use pasted plates similar to thick plate pasted batteries. The key difference lies in the electrolyte. In Gel batteries, the electrolyte is mixed with a silica gel compound, forming a thixotropic gel. This gel-like consistency prevents the electrolyte from spilling or leaking even if the battery case is damaged.
AGM Batteries
AGM (Absorbed Glass Mat) batteries have a different approach. They use fiberglass separators between the positive and negative plates. The electrolyte is absorbed in these glass mat separators, creating a sponge-like structure. This design allows for efficient ion exchange during charge and discharge cycles.
Comparison of Lead Acid Battery Types: Flooded, VRLA (AGM, Gel)
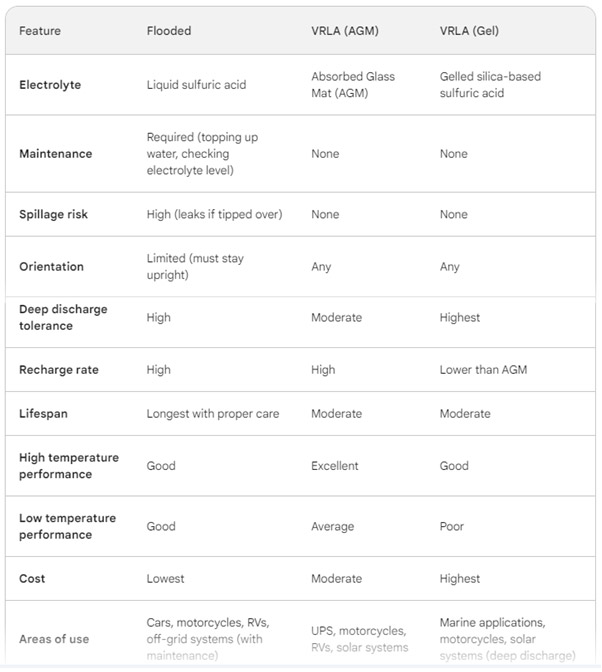
In Summary
For stationary applications, flooded batteries offer the best value for money, but require the most maintenance. VRLA ( AGM and Gel) batteries are generally safer than flooded batteries due to their spill-proof design.
AGM batteries are a good compromise between flooded and GEL batteries in terms of price, performance and maintenance.
Gel batteries are the most expensive but offer the best deep discharge tolerance and vibration resistance.
Modern Inverters and UPS Systems: Battery Charging Stages
1. Two-Stage Charging
I. Bulk Charging: Initial rapid charging to replenish most of the battery’s capacity.
II. Float Stage Charging: Maintains a constant voltage to keep the battery fully charged.
2.Three-Stage Charging
I. Bulk Charging: Rapid initial charge.
II. Absorption Charging: Gradual charging to reach full capacity.
III. Float Charging: Maintains the battery at optimal levels.
In the BULK charging stage, batteries are charged to 80% capacity at constant current, while increasing the voltage up to a maximum of 14.5V. The maximum charging current can be up to 10% of the AH capacity. During this stage, ensure that the electrolyte temperature does not exceed 50°C. If electrolyte temperature goes up above this, switch off charger and allow battery to cool down
[Caution] : For POWERCEL C10 Batteries, we recommend the normal charging to be limited to maximum 8% of the battery AH capacity.
In the ABSORPTION stage, the remaining 20% of capacity is charged at constant voltage of 14.5 volts and decreasing the current until the battery is fully charged. Check the electrolyte specific gravity to ensure full charging. For POWERCEL tubular batteries, the fully charged battery will have a specific gravity between 1.240 and 1.250. If gravity is higher, add DM water and reduce gravity to below 1.250
In the FLOAT charging stage when the battery reaches a lower voltage of 13.8 volts, a low current of 200 to 300 milliamps can be given to compensate for the battery self-discharge. This stage is normally used to maintain a fully charged condition of battery indefinitely when not discharged.
3. Equalization Charging – The Fourth Stage of Charging
This is occasional high-voltage charging to balance cell voltages. Equalization charging is essentially a controlled over charging process.
The electrolyte in a flooded battery, particularly in tower type stationary battery, stratifies over time, if not cycled occasionally. For the charged stratified batteries, the acid electrolyte concentration will be higher at the bottom (sp. gr. much above 1.240) and lower (sp. gr. Lower than 1.240 ) at the top of the battery.
Batteries tend to stratify if kept at a low charge (below 80% charge) and never have the opportunity to receive a full charge. This is called acid stratification and acid stratification reduces the overall capacity of the battery.
An equalization charging can effectively reduce stratification of flooded tall tubular batteries. In equalization charging, the voltage is brought up above typical peak charging voltage of 14.5 volts, up to 16 volts for a 12 volts battery. The charging at this voltage level is continued to the gassing stage and then held there for a fixed, but limited period. This speeds up the reaction in the entire battery, equalizing the strength of the electrolyte uniformly across. This controlled over voltage charging may also help to dissolve any loose sulfation formed on the battery plates. During the equalization charging the electrolyte temperature is to be kept below 50°C to avoid plate damage.
Important Note:
Remember, using a good quality charger is crucial for maximizing battery lifespan. For POWERCEL batteries, the normal full charge upper voltage should be 14.5 volts. Exceeding this voltage can lead to overheating, electrolyte gassing and faster water evaporation from the battery.
Battery Capacity Degradation: Factors
Over time, battery capacity undergoes degradation due to sulfation (formation of lead sulfate crystals) and shedding of active material. The extent of this degradation is influenced by several critical parameters:
1. Charging/Discharging Regime
The battery’s history of charge and discharge cycles significantly impacts its capacity.
Below graph illustrates the evolution of battery life cycles vs discharge rate. The typical useful life of a deep discharge battery is around 1000 cycles. However, if the depth of discharge is maintained at less than 50% of capacity, the battery life time can be increased to much more than 1000 cycles. Remember, understanding these factors helps optimize your battery performance and prolong its lifespan.
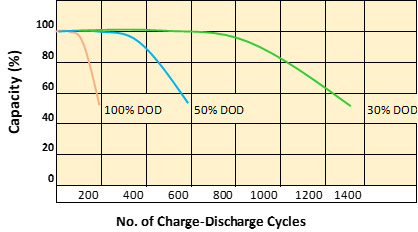
Graph: Relationship between battery capacity, depth of discharge and life of battery
2. The Quality of charging
The quality of charging also plays an important part in determining battery lifetime. Overcharging or undercharging the battery results in either the shedding of active material or the sulfation of the battery, thus greatly reducing battery life.
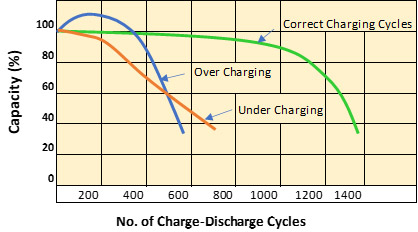
Graph: impact of charging regime of battery capacity
Undercharging occurs when the charger fails to restore the battery to its full charge after use. Continuous operation of the battery in a partial state of charge (SoC) or storing it in a discharged state for long, leads to the formation of lead sulfate (sulfation) on the battery plates. It is also sometimes referred to as plate hardening.
Sulfation is the reason for premature failure of 80% of the batteries. If sulfation can be prevented, we can extend useful life of a battery by more than double of the normal expected life.
Lead sulfate (PbSO4) formation is a normal process in the lead acid battery charge -discharge cycle. As the battery is discharged, the electrodes become coated with lead sulfate and the acid electrolyte becomes weaker and indicated as lower specific gravity. When the battery is charged back immediately after discharge, the lead sulfate coating on the electrodes is removed, converted back to lead and the acid electrolyte becomes stronger by generation of sulfuric acid from lead sulfate.
When battery is kept discharged for long or not charged fully every time, lead sulfate formed will stick to the plates and hardens. This prevents efficient flow of current to the plates, making the battery partially dead or fully unusable.
There are electronic charging devices for removing hard sulfation, but the best practice is preventing formation by proper battery care and recharging after a discharge cycle.
Overcharging or continuous charging without end of charge upper voltage cut off causes accelerated corrosion of the positive plates, excessive water loss and in some cases causes higher temperatures developing in the battery cells. A good quality charger or a charger circuit for inverter or UPS is essential to prevent over charging.
3. Operating Temperature
The battery’s average operating temperature over its lifespan plays a crucial role in it’s life. Although the capacity of a lead acid battery is reduced in low temperature operation, high temperature operation increases the aging rate of the battery.

Graph: Relationship between AH capacity, temperature and lifetime of deep-cycle battery.
4. Under-watering
In lead-acid batteries, water is lost during the charging process. If the electrolyte level drops below the top of the plates, irreparable damage may occur. When your battery charges, the electrolyte heats up and some of the water evaporates. Also, during a process called electrolysis, the water breaks down into hydrogen and oxygen gases that dissipate and the electrolyte level in the battery lowers over time. If the electrolyte level is too low, the plates in the battery cells are exposed and will suffer irreversible damage. In addition, the sulfuric acid will be more concentrated and leads to rapid grid corrosion as well.
5. Over-watering
Excessive watering of a battery results in additional dilution of the electrolyte, resulting in reduced battery performance. Add water to your battery after it has been fully charged. Never add water when the battery is in discharged state. To prevent this situation, check electrolyte levels regularly. In stationary batteries, ceramic vent plug electrolyte level indicators are provided to show the minimum and maximum electrolyte levels. Add distilled water (also called deionized water or demineralized water) when electrolyte level nears the lower limit.
Lead-acid batteries also have a high, as much as 98 percent, rate of recycling which helps offset concerns about the toxicity of their materials. Once the battery can no longer be recharged, the lead from the electrodes and the plastic from the battery housing are recycled, and the sulphuric acid electrolyte is usually neutralized. Lead acid batteries are manufactured from 60 to 80 percent recycled materials.
Caution:
Do not dispose the old battery to a non-authorized person. As per Govt. of India Notification, “It shall be the responsibility of the consumer to ensure that used batteries are not disposed of in any manner other than depositing with the dealer, manufacturer, re-conditioner or at the designated collection centres”
